Overview
The surfaces of cells are covered with a dense layer of glycoproteins, glycolipids, and proteoglycans, which together make up the glycocalyx. These glycoconjugates bind to growth factors, enzymes, and extracellular matrix proteins, and thereby participate in a wide variety of biological phenomena related to cell differentiation, proliferation and migration, morphogenesis, and normal and pathophysiology. To a large extent these interactions are determined by the structure of the polysaccharide chains (glycans) that distinguish the various subclasses of glycoconjugates. The assembly of these molecules involves many enzymes, substrates and cofactors and differs from the assembly of nucleic acids and proteins in not requiring a template. Understanding how cells organize the assembly process to bring about cell-type specific glycan expression and biological responses is a major problem in modern cell biology.
Research in the Esko lab utilizes a combination of chemistry, cell biology and genetics to understand the structure, assembly and function of sulfated glycosaminoglycans found on proteoglycans. The glycosaminoglycans include hyaluronan, heparan sulfate, chondroitin/dermatan sulfate, and keratan sulfate. Studies of mutant cell lines and mice bearing conditional and systemic mutations allow us to analyze glycan function in normal physiology and disease. Current work arranged by systems include:
- Proteoglycan Structure and Metabolism: We have an ongoing program to characterize mouse strains lacking specific sulfotransferases and glycosyltransferases involved in heparan and chondroitin/dermatan sulfate assembly. These studies also involve the development of methods to characterize the structure of the glycosaminoglycan chains.
- Proteoglycans and Infectious Disease: A major effort in the lab focuses on the impact of heparan sulfate on infectious disease. These studies involve assessment of the vascular glycoproteome remodeling after bacterial challenge. Another set of studies focus on altering heparan sulfate in endothelial cells and myeloid cells to determine how these changes affect infection and inflammation. A third area concerns the impact of natural variation in heparan sulfate content and composition on infection.
- Proteoglycans and the Eye: This area involves collaborative studies with Dr. Christopher Toomey, a retinal surgeon at UCSD. Specifically, we are interested in the role of glycosaminoglycans in the retina in the formation of drusen, deposits of protein and lipid in Bruch’s membrane, which can lead to early stages of macular degeneration. Methods are being developed to prevent or reverse these drusen deposits.
The lab currently consists of 3 postdoc researchers, 1 graduate student, 2 technicians, and 2 undergraduates. We have lab meeting every week (Thursdays at 8:00 am), a joint meeting with Professor Toomey’s lab (every other Tuesday at 8:30 am), a journal club focused on Current Literature in Glycobiology (BIOM 246, weekly on Friday), and weekly submeetings to coordinate projects with collaborators on and off campus. There are several classroom opportunities to learn more about glycans (see Courses). Trainees receive classical training in the chemistry and biochemistry of glycans and modern training in genetics, cell biology and physiology. Stop by, my door is always open.
Proteoglycan Structure and Metabolism
Virtually all animal cells carry proteoglycans on their plasma membrane and secrete them into the surrounding extracellular matrix. The proteoglycans consist of a protein core and one or more covalently linked glycosaminoglycan chains, such as heparan sulfate (which is related in structure to the anticoagulant heparin) or chondroitin sulfate/dermatan sulfate. A large family of enzymes install sulfate groups at various positions along the chains or epimerize the uronic acids, creating binding sites for various ligands, including growth factors, proteases and their inhibitors, lipolytic enzymes and plasma apolipoproteins, and extracellular matrix proteins. The importance of these interactions is exemplified by the profound pathophysiological phenotypes in mice and humans bearing mutations in the core proteins or the biosynthetic enzymes responsible for assembly of the chains.
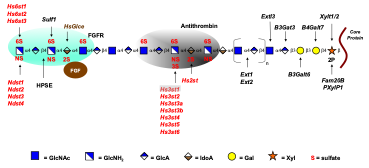
Ongoing projects in the lab include creation of conditional mutants in mice in order to study the function of heparan sulfate and chondroitin sulfate proteoglycans in different physiological systems. We are particularly interested in Hs3st enzymes, a family of glucosaminyl 3-O-sulfotransferases that modify heparan sulfate by the installation of a sulfate group at C3 of N-sulfoglucosamine units. One of these enzymes, Hs3st1, endows the chains with the capacity to bind and activate antithrombin, a serine protease inhibitor that is expressed in all epithelial and endothelial cells. We are currently studying this enzyme in the context of tumor formation and as an anti-inflammatory factor in tissues.
A technical challenge in this field is the development of methods for structural analysis of the chains. We previously developed a highly sensitive method for analyzing the disaccharide subunit structure of all glycosaminoglycan chains based on stable isotope tagging with aniline and quantitative mass spectrometry. These highly sensitive techniques are being adapted to other methods to depolymerize the chains, creating a toolbox of orthogonal techniques to study overall structure of the chains.
Relevant Papers
Thacker, B.E., Xu, D., Lawrence, R. and Esko, J.D. (2014) Heparan sulfate 3-O-sulfation: A structure in search of a function. Matrix Biol. 35:60-72. PMID:24361527, PMCID:4039620.
Thacker, B.E., Seamen, E., Lawrence, R., Parker, M.W., Xu, Y., Liu, J., Vander Kooi, C.W., and Esko, J.D. (2016) Expanding the 3-O-sulfate proteome - Enhanced binding of neuropilin-1 to 3-O-sulfated heparan sulfate modulates its activity. ACS Chem. Biol. 11:971-980. PMID:26731579, PMCID:5450942.
Thacker, B.E., Thorne, K.J., Cartwright, C., Park, J., Glass, K., Chea, A., Kellman, B.P., Lewis, N.E., Wang, Z., Di Nardo, A., Sharfstein, S.T., Jeske, W., Walenga, J., Hogwood, J., Gray, E., Mulloy, B., Esko, J.D. and Glass, C.A. (2022) Multiplex genome editing of mammalian cells for producing recombinant heparin. Metab. Eng. 70:155-165. PMID:35038554.
Spliid, C.B., Mehta, S., Fuster, M.M., Martino, C., Morris, C.L., Lee, N., Florentino, I., Tong, K., Liu, L., Ackermann, G., Knight, R., Esko, J.D. and De Mendoza, T.H. (2024) Diversity of human salivary heparan sulfate. Glycobiology 34, cwae084. PMID: 39361890
Clausen, T.M., Weiss, R.J., Tremblay, J.R., Kellman, B.P., Coker, J., Dworkin, L.A., Rodriguez, J.P., Chang, .I.M., Chen, T., Padala, V., Karlsson, R., Song, H., Peck, K.L., Ogawa, S., Sandoval, D.R., Joshi, H.J., Wang, G., Ferguson, L.P., Bhalerao, N., Moores, A., Reya, T., Sander, M., Caffrey, T.C., Grem, J.L., Aicher, A., Heeschen, C., Le, D., Lewis, N.E., Hollingsworth, M.A., Grandgenett, P.M., Bellis, S.L., Miller, R.L., Fuster, M.M., Dawson, D.W., Engle, D.D. and Esko, J.D. (2025) Antithrombin-binding heparan sulfate is ubiquitously expressed in epithelial cells and suppresses pancreatic tumorigenesis. Clin. Invest. 16: e184172.
Proteoglycans and Infectious Disease
 A major effort in the lab focuses on the impact of infection on the glycocalyx surrounding endothelial cells. Previously, we published a systems-wide approach using data-independent acquisition (DIA) mass spectrometry to track the progression of bacterial sepsis induced by methicillin-resistant Staphylococcus aureus (MRSA) in the vasculature leading to organ failure. This approach was based on vascular tagging, in which mice were injected with a membrane impermeable biotinylation agent (NHS-sulfo-biotin) to tag the vascular proteome. We demonstrated the profound proteome remodeling triggered by MRSA sepsis over time and across different organs. To determine if the vascular proteome changes dependent on the pathogen, we applied the in vivo biotinylation method to mice infected with Streptococcus pneumoniae (SPN). The data will then be compared to the plasma proteome to determine if glycocalyx associated proteins are shed into the circulation, and to similar datasets generated previously from animals infected with MRSA to determine if these two organisms show unique vascular proteomic responses.
A major effort in the lab focuses on the impact of infection on the glycocalyx surrounding endothelial cells. Previously, we published a systems-wide approach using data-independent acquisition (DIA) mass spectrometry to track the progression of bacterial sepsis induced by methicillin-resistant Staphylococcus aureus (MRSA) in the vasculature leading to organ failure. This approach was based on vascular tagging, in which mice were injected with a membrane impermeable biotinylation agent (NHS-sulfo-biotin) to tag the vascular proteome. We demonstrated the profound proteome remodeling triggered by MRSA sepsis over time and across different organs. To determine if the vascular proteome changes dependent on the pathogen, we applied the in vivo biotinylation method to mice infected with Streptococcus pneumoniae (SPN). The data will then be compared to the plasma proteome to determine if glycocalyx associated proteins are shed into the circulation, and to similar datasets generated previously from animals infected with MRSA to determine if these two organisms show unique vascular proteomic responses.
During the course of these studies, we discovered a dramatic accumulation of a novel acute phase protein called PRG4, whose expression is rapidly upregulated in bacterial infection, and that physically associates with the surface of injured endothelium and occluding thrombi. PRG4, also known as proteoglycan 4 and lubricin, is a heavily glycosylated mucin-like protein required for lubrication of articular surfaces, pericardial tissue and the eyelid-corneal interface. PRG4 is secreted by liver hepatocytes and circulates in plasma, but the function of this hepatic source and its involvement in human disease remain unknown. PRG4 accumulates in the plasma of human septic patients and in mice, where it reaches surprisingly high levels, akin to major acute-phase proteins.
Notably, addition of rhPRG4 to plasma significantly extended adjusted prothrombin time (aPTT) in a dose-dependent manner, nearly doubling the aPTT when the concentration of rhPRG4 neared the average values found in septic plasma. In contrast, the prothrombin (PT) assay, which measures the extrinsic pathway of coagulation, was insensitive to the presence of rhPRG4. This selective inhibitory effect of PRG4 on aPTT but not on PT measurements resembles clinical findings in patients with anti-phospholipid syndrome (APS), an autoimmune condition characterized by the presence of autoantibodies against phospholipids and phospholipid-binding proteins, resulting in an increased risk for thrombosis and pregnancy-related complications. Our findings indicate that PRG4 regulates the coagulation pathway under inflammatory conditions, like that manifested in infection.
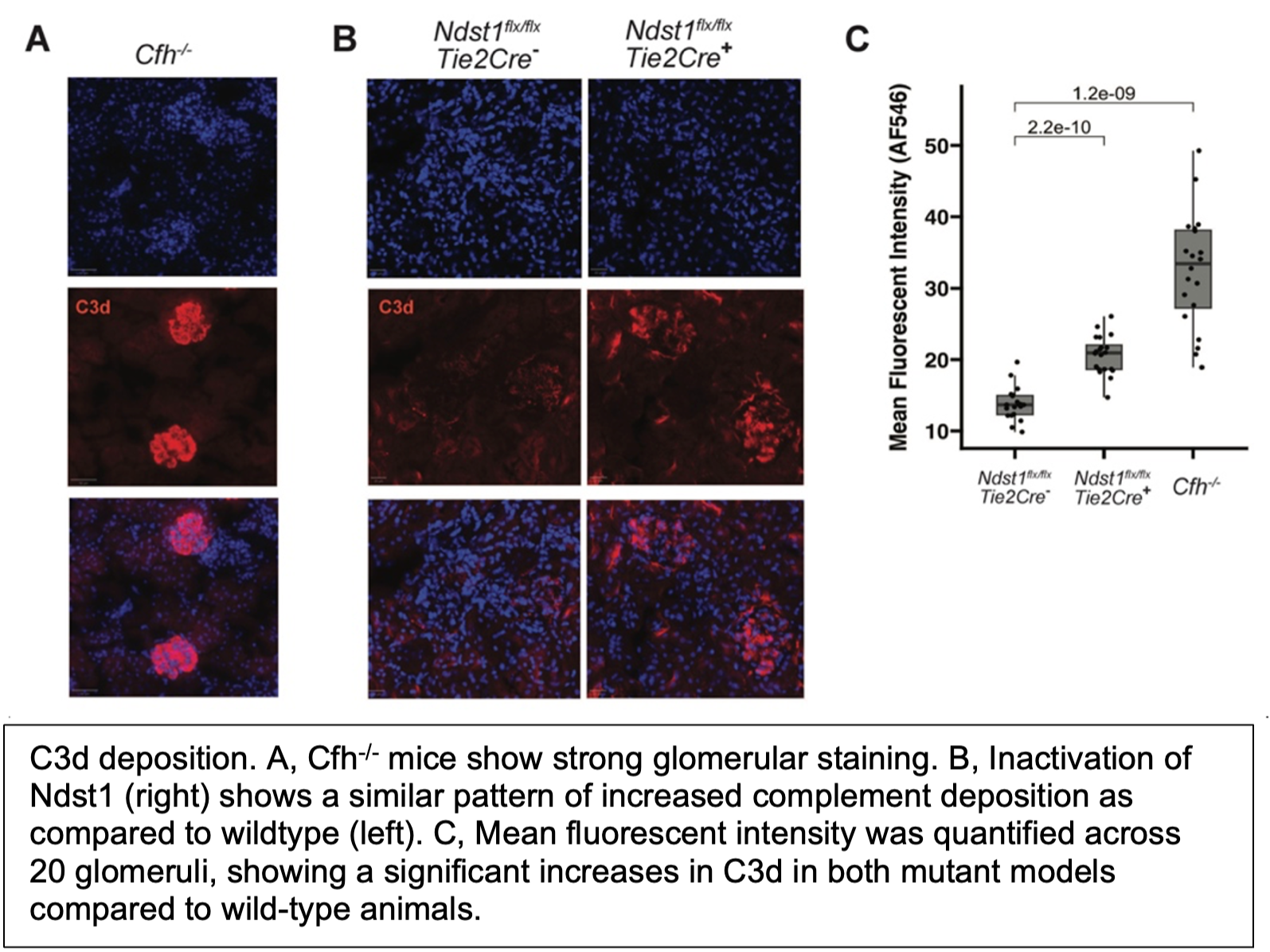
Another area of study relevant to infection concerns the activation of the complement system, our first line of defense to invading pathogens. The complement system is regulated by Factor H and Factor H related proteins, which bind heparan sulfate. To understand the physiological relevance of these interactions, we have examined complement deposition in animals containing genetic alterations in heparan sulfate in endothelial cells. Aged animals show deposition of complement C3 components in the glomeruli of the kidney, suggesting that altered binding of one or more the Factor H family of proteins to heparan sulfate plays a role in regulating complement activation. To our knowledge, this model is the first to replicate the C3 deposition observed in C3 Glomerulopathy without altering complement proteins directly.
Relevant Papers
Toledo, A.G., Golden, G., Campos, A.R., Cuello, H., Sorrentino, J., Lewis, N., Varki, N., Nizet, V., Smith, J.W. and Esko, J.D. (2019) Proteomic atlas of organ vasculopathies triggered by Staphylococcus aureus sepsis. Commun. 10:4656. PMID:31604940 PMCID:6789120.
Golden, G.J., Toledo, A.G., Marki, A., Sorrentino, J.T., Morris, C., Riley, R.J., Spliid, C., Chen, Q., Cornax, I., Lewis, N.E., Varki, N., Le, D., Malmström, J., Karlsson, C., Ley, K., Nizet, V. and Esko, J.D.. (2021) Endothelial heparan sulfate mediates hepatic neutrophil trafficking and injury during Staphylococcus aureus mBio 12:e0118121. PMID:34544271 PMCID:8546592.
Sorrentino, J.T., Golden, G.J., Morris, , Painter, C., Nizet, V., Campos, A.R., Smith, J.W., Karlsson, C., Malmström, J., Lewis, N.E., Esko, J.D. and Toledo, A.G. (2022) Vascular proteome responses precede organ dysfunction in a murine model of Staphylococcus aureus bacteremia (2022) mSystems 7:e0039522. PMID:35913192 PMCID:9426442.
Painter, C.D., Sankaranarayanan, N.V., Nagarajan, B., Mandel Clausen, T., West, A.M.V., Setiawan, N.J., Park, J., Porell, R.N., Bartels, P.L., Sandoval, D.R., Vasquez. G.J., Chute, J.P., Godula, K., Vander Kooi, C.W., Gordts, P.L.S.M., Corbett, K.D., Termini, C.M., Desai, U.R. and Esko J.D. (2024) Alteration of neuropilin-1 and heparan sulfate interaction impairs murine B16 tumor growth. ACS Biol. 19:1820-1835. PMID:39099090 PMCID:11334110.
Toledo, A.G., Golden, GJ., Cummings, R.D., Malmstrom, J. and Esko, J.D. (2025) Endothelial glycocalyx turnover in vascular health and disease: rethinking endothelial dysfunction. Annu. Rev. Biochem. 94:561 PMID:40132227.
Proteoglycans and the Eye
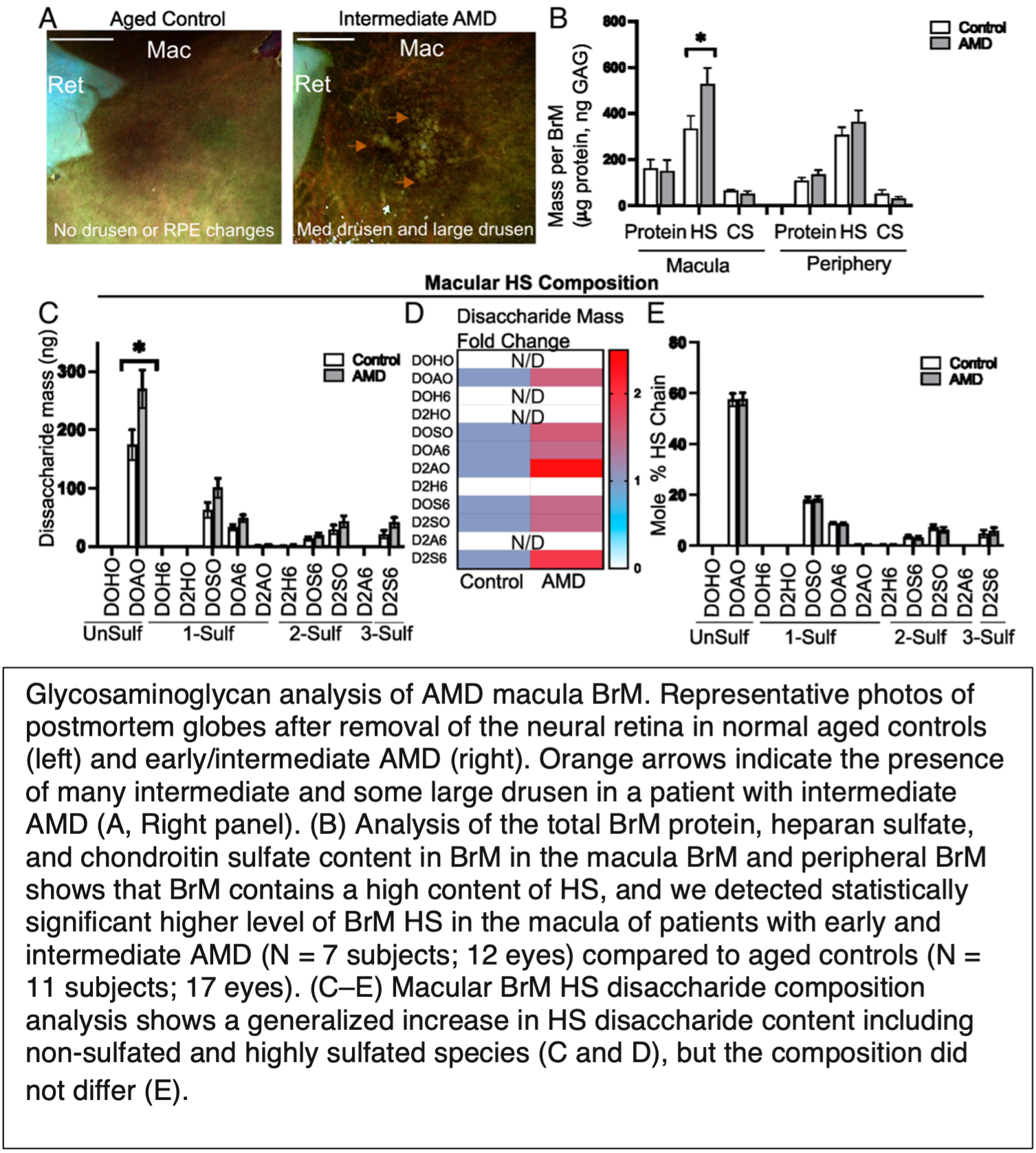 This area of research currently involves collaborative studies with Dr. Christopher Toomey, MD/PhD, a retinal surgeon at UC San Diego. Specifically, we are interested in the role of glycosaminoglycans in the retina in the formation of drusen, which are deposits of protein and lipid in Bruch’s membrane. Drusen deposition in the macula of the eye is a hallmark of early stages of macular degeneration. Drusen appears to contain lipoprotein-like structures resembling high density lipoproteins (HDL) in the plasma, but the drusen associated particles appear to contain unique lipids and apolipoproteins. The mechanism of lipoprotein retention in BrM is unknown. We recently showed that BrM heparan sulfate accumulates in human eyes from patients with AMD. Scanning electron microscopy of postmortem AMD tissue revealed aggregates of lipoprotein-like particles on the retinal pigmented epithelium side of Bruch's membrane adjacent to heparan sulfate. We also showed that heparin displaces lipoproteins from human BrM and that heparan sulfate in human BrM immobilized to quartz crystal microbalance biosensor (QCM) chips mediate lipoprotein binding. Current studies focus on understanding how variation in the structure of heparan sulfate across individuals and regions of the retina could influence lipoprotein retention and development of AMD.
This area of research currently involves collaborative studies with Dr. Christopher Toomey, MD/PhD, a retinal surgeon at UC San Diego. Specifically, we are interested in the role of glycosaminoglycans in the retina in the formation of drusen, which are deposits of protein and lipid in Bruch’s membrane. Drusen deposition in the macula of the eye is a hallmark of early stages of macular degeneration. Drusen appears to contain lipoprotein-like structures resembling high density lipoproteins (HDL) in the plasma, but the drusen associated particles appear to contain unique lipids and apolipoproteins. The mechanism of lipoprotein retention in BrM is unknown. We recently showed that BrM heparan sulfate accumulates in human eyes from patients with AMD. Scanning electron microscopy of postmortem AMD tissue revealed aggregates of lipoprotein-like particles on the retinal pigmented epithelium side of Bruch's membrane adjacent to heparan sulfate. We also showed that heparin displaces lipoproteins from human BrM and that heparan sulfate in human BrM immobilized to quartz crystal microbalance biosensor (QCM) chips mediate lipoprotein binding. Current studies focus on understanding how variation in the structure of heparan sulfate across individuals and regions of the retina could influence lipoprotein retention and development of AMD.
Relevant Papers
Pan, Y., Woodbury, A., Esko, J.D., Grobe, K., and Zhang, X. (2006) Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development 133:4933-4944. PMID:17107998
Coulson-Thomas, V.J., Chang, S.H., Yeh, L.K., Coulson-Thomas, Y.M., Yamaguchi, Y., Esko, J., Liu, C.Y. and Kao, W. (2015) Loss of corneal epithelial heparan sulfate leads to corneal degeneration and impaired wound healing. Ophthalmol. Vis. Sci. 56:3004-3014. PMID:26024086, PMCID:4432551.
Tao, C., Makrides, N., Chuang, J.Z., Wu, Y., Brooks, S.E., Esko, J.D., Sung, C.H. and Zhang, X. (2022) Chondroitin sulfate enhances the barrier function of basement membrane assembled by heparan sulfate. Development 149:dev200569. PMID:35608020 PMCID:9107513
Chen, J.S., Esko, J.D., Walker, E., Gordts, P.L.S.M., Baxter, S.L. and Toomey, C.B. (2025) High Density Lipoproteins associate with Age-Related Macular Degeneration in the All of Us Research Program. Ophthalmology. 132:684-691. PMID:39756691.
Toomey, C.B.; Pflugmacher, S., Park, K., Pihl, J., Weiser Novak, S., Rodriguez, J., Jung, J., Hauer, J., Huang, A., Boassa, D., Handa, J.T., Astrup, T., Saraswat, M., Gordts, P.L.S.M. and Esko, J.D. (2025) Bruch’s membrane heparan sulfate retains lipoproteins in the early stages of age-related macular degeneration. Proc. Natl. Acad. Sci. USA, 122:e2500727122. PMID:40512794