About Us
Autophagy and quality control
The goal of our research program is to understand how homeostasis is maintained in the endoplasmic reticulum
The endoplasmic reticulum (ER) is a major site of protein biogenesis in the cell. When errors in protein assembly occur, misfolded proteins are extruded from the ER and degraded in the cytosol. Many aggregation-prone, disease-causing proteins, however, cannot be retrotranslocated across the ER membrane and must be cleared from the ER by autophagy. Recent studies in the lab have focused on understanding how the mammalian ER autophagy (called ER-phagy) machinery recognizes this class of misfolded proteins and targets them to the lysosome for degradation. These studies have uncovered novel machinery that packages ER subdomains, that contain misfolded cargos, into autophagosomes (Cui et al. 2019 Science 365:53-60; Parashar et al. eLife 2021 doi:10.7554/eLife.71642). Autophagosomes are distinct organelles that deliver deleterious or damaged cellular components to lysosomes for degradation. Defects in ER autophagy have been linked to metabolic disorders and neurodegenerative diseases, including hereditary spastic paraplegias.
Projects in the lab are aimed at addressing the following questions:
- The role that ubiquitination plays in ER autophagy.
- The role that ER membrane contact sites play in autophagy
- The role that lipid droplets (LD) play in ER autophagy. LDs are conserved ER-derived organelles that store excess fatty acids (FAs), such as triacylglycerol and cholesterol esters, in the form of neutral lipids.
- the connection between mitophagy (autophagic degradation of mitochondria) and ER-phagy
- The role that ER structure plays in ER autophagy (see posted Figure and legend)
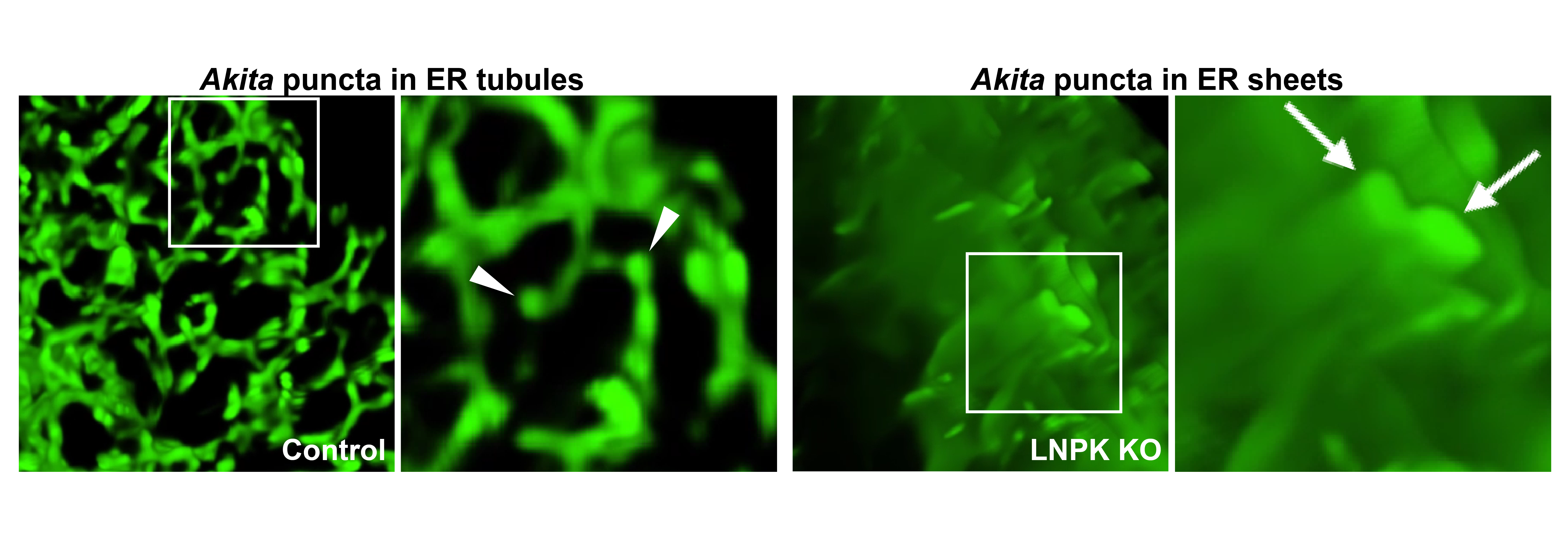 Legend: The Figure shows misfolded proinsulin, Akita, in ER tubules (left) and proliferated sheets (right). Akita forms condensates (puncta in images) that enlarge when ER tubules are converted to sheets in Lunapark knock-out (LNPK KO) cells. LNPK stabilizes three-way junctions, and its absence ER sheets become more abundant. The image on the left is a maximum intensity projection showing Akita condensates in tubules. The inset is expanded on the right in the 3D reconstruction, and arrowheads point to the Akita condensates/puncta in tubules. The image on the far right is a 3D reconstruction of Akita puncta (see arrows) that have expanded in the ER sheets that proliferate in LNPK KO cells.
Legend: The Figure shows misfolded proinsulin, Akita, in ER tubules (left) and proliferated sheets (right). Akita forms condensates (puncta in images) that enlarge when ER tubules are converted to sheets in Lunapark knock-out (LNPK KO) cells. LNPK stabilizes three-way junctions, and its absence ER sheets become more abundant. The image on the left is a maximum intensity projection showing Akita condensates in tubules. The inset is expanded on the right in the 3D reconstruction, and arrowheads point to the Akita condensates/puncta in tubules. The image on the far right is a 3D reconstruction of Akita puncta (see arrows) that have expanded in the ER sheets that proliferate in LNPK KO cells.
Contact Us
|
Susan Ferro-Novick, sfnovick@ucsd.edu Department of Cellular and Molecular Medicine Office Phone: (858) 246-0466 |
|
